Welcome to BrainFrontier - Your weekly newsletter cutting through the noise in neuroscience and psychedelic research. We track breakthrough studies, clinical trials, and market trends—and distill them into simple, actionable insights for investors, clinicians, and brain science enthusiasts.
What's inside this week:
🔬 [MAIN STORY TOPIC]
🧠 [SCIENCE HIGHLIGHT]
💼 [MARKET/INDUSTRY IMPLICATION]
👩⚕️ [CLINICAL RELEVANCE]
📊 [DATA/STATISTICS HIGHLIGHT]
BREAKTHROUGH ALERT
MDMA-Assisted Therapy for PTSD Nears FDA Approval After Phase 3 Triumph
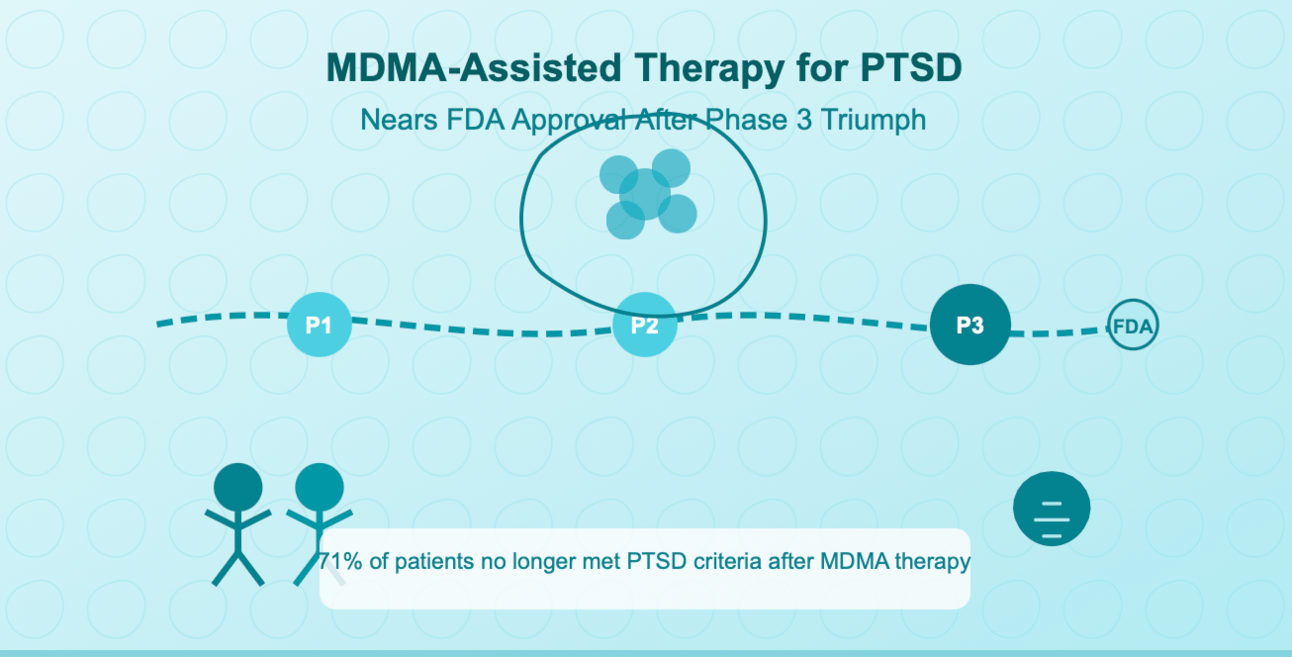
The Remarkable 71% Success Rate
A groundbreaking Phase 3 trial has confirmed what researchers have long suspected: MDMA-assisted psychotherapy can dramatically reduce or even eliminate severe PTSD symptoms. With the FDA expected to review the application this year, we could see the first psychedelic-assisted therapy reach mainstream medical use in U.S. history.
By the Numbers:
71% of patients no longer met PTSD criteria after MDMA therapy (vs. 47% with placebo)
3 supervised MDMA-assisted therapy sessions produced these results
0 serious adverse events reported
2024 expected FDA approval timeline
THE SCIENCE BEHIND THE BREAKTHROUGH
The Science Behind the Breakthrough
In a rigorous multisite, randomized Phase 3 clinical trial (MAPP2) published in Nature Medicine last September, 104 participants with moderate-to-severe PTSD received talk therapy with either MDMA or placebo over 18 weeks.
The results were striking:
After three supervised MDMA-assisted therapy sessions (plus preparatory and integrative therapy), 71.2% of participants no longer met PTSD diagnostic criteria, compared to 47.6% in the psychotherapy-plus-placebo group.
This confirms the results of an earlier Phase 3 trial, firmly establishing the treatment's efficacy.
How Does It Work?
MDMA (3,4-methylenedioxymethamphetamine) appears to work by:
Increasing serotonin and oxytocin release
Enhancing emotional processing and fear extinction during therapy
Creating a sense of safety that allows patients to process trauma without being overwhelmed by fear
Dr. Jennifer Mitchell, the trial's lead author, noted this non-avoidant processing as key to "Rewire the brain," helping patients reconsolidate traumatic memories with significantly less distress.
Based on these compelling outcomes, MAPS PBC (the sponsor) is compiling data from 18 Phase 2/3 studies to submit a New Drug Application to the FDA.
WHAT THIS MEANS FOR…
What This Means For...
🧠 Patients & Clinicians
A powerful new option for PTSD sufferers, especially those not helped by existing treatments. Therapists gain a potent tool to help patients process trauma in ways not possible with conventional approaches.
🔬 Psychiatry & Research
This success "nears the tipping point" for accepting psychedelic therapy in mainstream mental health. It validates years of research and could accelerate trials of psilocybin, LSD, and other psychedelics for depression, anxiety, and addiction.
⚖️ Regulators & Policy
An FDA approval would necessitate rescheduling MDMA from Schedule I, removing legal barriers for medical use. This may prompt regulators worldwide to reevaluate policies on psychedelics.
💼 Industry & Investors
As the first commercial psychedelic therapy, this creates a framework for clinics, training programs, and insurance coverage. Expect increased investment in psychedelic startups as treatment moves from non-profit trials to broader markets.
EXPERT PERSPECTIVE
Expert Perspective
"This treatment approach has the potential to transform the lives of people suffering from PTSD, a condition that affects millions worldwide and has proven difficult to treat with existing therapies."
WHAT’S NEXT
What's Next?
MAPS PBC is preparing its FDA submission for 2024
Companies are scaling up pharmaceutical-grade MDMA production
Therapist training programs are expanding to meet anticipated demand
Investors are watching closely for regulatory frameworks that will shape the market
FURTHER READING
Further Reading
BrainFrontier cuts through the noise in neuroscience and psychedelic research. We track breakthrough studies, clinical trials, and market trends—and distill them into simple, actionable insights.
Subscribe to stay ahead of the next big discovery without drowning in research.
© 2025 BrainFrontier. All rights reserved.